- Home
- Herbal Remedies
- Plant Tannins
Plant Tannins as Antibacterial & Antifungal Agents
Posted 2/16/2020
Written by Pharmaceutical Scientist Dr. Harmeet Kaur, PhD
Plant tannins have activity against bacteria and yeast in a laboratory but do they work well in the body? The answers are in the article below.
The term plant tannins was first introduced in 1796 by Seguin to denote compounds present in plant extracts, which can combine with proteins of animal hides; prevent their putrefication and convert them into the leather. Tannins are water-soluble polyphenol derivatives naturally synthesized and accumulated by higher plants as secondary metabolites of molecular weights in the range of 500 and 3000 Da. In complexes with alkaloids, saccharides, and proteins, the molecular weight can go up to 30,000 Da.
The chemical structure of tannins mainly depends on the plant species from which they are obtained. They differ from another polyphenolic compound as their ability to precipitate proteins (astringent action). Presently, over 8000, different tannins from various plant parts have been isolated and chemically characterized. Due to medicinal properties, these are employed as hemostatic, antidiarrheal, and antibacterial compounds [1,2].
Classification of Plant Tannins
Based on standard structural features, the tannins can be classified into two main categories, hydrolysable tannins (HTs) and nonhydrolysable or condensed tannins (CTs). The HTs are generally partial or total esters of polyol moiety such as sugars with polyphenolic compounds like gallic acid (gallotannins; GTs) and ellagic acid, which is lactone form of hexahydroxydiphenic acid (elagllitannins; ETs). The GTs are mainly extracted from sumac (Rhus coriaria), tara (Caesalpinia spinosa), and gallnuts (Quercus infectoria and Rhus semialata).
Tannic acid is the simplest gallotannin which is formed by a pentagalloyl glucose molecule esterified by five gallic acid units. The ETs, are mainly present in chestnut wood (Castanea sativa), oak wood (Quercus robur, Quercus petraea and Quercus alba), and myrobalan (Terminalia chebula). Castalagin, castalin, casuarictin, grandinin, roburin A, tellimagrandin II, punicalagin and punicalin are some of the examples of ETs. HTs are generally hydrolyzed by weak acids or weak bases to produce carbohydrates and phenolic acids [3].
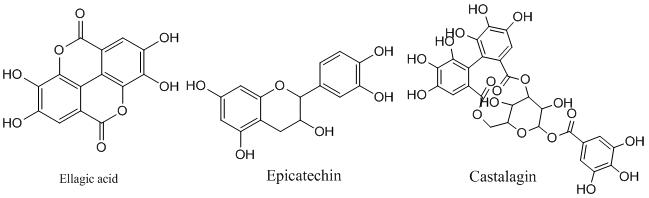
The CTs, also called procyanidins are polymers of flavonoids approximately 2 to 50 units joined by C-C bonds, which are not hydrolyzed by enzymes like tannases. These are further classified as proanthocyanidin and profisetinidin. Proanthocyanidins are naturally present in the skins and seeds of grapes (Vitis vinifera), and a common constituent of red wines. Profisetinidins are isolated from the mimosa (Acacia mollissima) and quebracho wood (Schinopsis lorentzii). CTs are much more resistant to microbial degradation in comparison to the HTs and exhibit stronger antiviral, antibacterial, and antifungal activity.
The chemical structure of plant tannins determines their biological activity. Moreover, tannins can act as potential: biological antioxidant, metal ion chelating agent, or depending on its concentration, as a complexing or precipitating agent (in low concentrations as a complexing, and in high as a precipitating agent). Recently the third class of tannins has been identified, the phlorotannins, present in many species of dark algae [4].
Role of Plant Tannins as Antibacterial Agents
Several studies have been performed from time to time to prove the
antimicrobial potential of plant tannins. The corilagin and terchebulin,
ETs isolated from Terminalia chebula has demonstrated moderate
antimicrobial activity against multidrug-resistant Acinetobacter
baumannii with a minimum inhibitory concentration (MIC) of around 500
μg/mL. This study has also identified
that the compound norwogonin from Scutellaria
baicalensis was highly
effective against A.
baumanii [5].
In a similar study, the 50% aqueous ethanolic extracts of
Acalypha wilkesiana and Acalypha hispida leaves having constituents
of gallic acid, corilagin and geraniin have shown good antimicrobial
effect against Escherichia
coli, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus
subtilis and Candida
pseudotropicalis [6].
In another study, the corilagin has demonstrated
antimicrobial activity against E.
coli, S.
aureus and Candida
albicans and found that
inhibition of E. coli
and C. albicans
by corilagin is due to disruption of cell membrane and enhanced
permeability. Further the activity against S.
aureus is due to action
on the proteins Fib, Sae R and Sar S. Protein Tye 7p was revealed to
be the possible target in C.
albicans [7].
Punicalagin
is an ET, found in α and β forms in pomegranates (Punica granatum).
Punicalagin, tannic acid, castalagin and geraniin have shown moderate
activity against genus Vibrio
and S. aureus
and weak activity against E.
coli. It has been
noticed that the punicalagin from Punica granatum fruits are more
potent against methicillin-resistant S.
aureus (MRSA) than the
punicalagin obtained from Terminalia citrina [8].
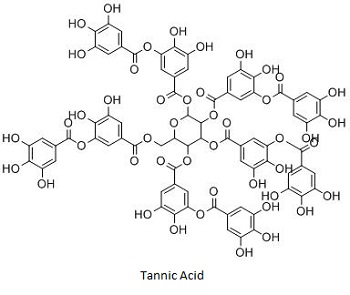
Tannic acid (an gallotannin) has
shown significant antibacterial action against S.
aureus. Tannic acid is
also known as a safe food additive present in many foods like grapes,
cocoa beans, and tea and has shown its inhibitory effect on the
growth of the intestinal bacteria like Clostridium
perfringens, Bacteroides fragilis, E. coli
and Enterobacter cloacae.
Mechanistically the tannic acid had greater binding efficiency with
iron, and thus, it may act as a siderophore to chelate iron from
medium and make iron unavailable to the microorganisms [9].
Funatogawa et al. evaluated nine HTs i.e. tellimagrandin I
(TG-I), tellimagrandin II (TG-II), acid-treated TG-I, acid-treated
TG-II, agrimoniin, geraniin, oenothein A, epigallocatechin gallate
(EGCG) and epicatechin (EC) for the bacterial membrane-disrupting
activity by labelling with carboxyfluorescein. All the plant tannins
have shown considerable membrane-damaging activity with the highest
activity of EGCG and minimal for EC. The same nine compounds have
also demonstrated good antibacterial activity towards Helicobacter
pylori but no activity
against E. coli,
which naturally found in the gastrointestinal tract of humans [10].
The plant tannin profile of Cytinus hypocistis and C.
ruber shown a significant amount of GTs, in particular
1-O-galloyl-β-D-glucose which have demonstrated activity against
three Gram-positive bacterial species (S.
aureus, S. epidermidis, Enterococcus faecium)
but no effect on Gram-negative strains (P.
aeruginosa and K.
pneumonia).
Interestingly, the suppressive activity of ethanolic extracts of
C. hypocistis and C.
ruber on biofilm
formation of S.
epidermidis has been
observed [11].
The CTs
from the stem barks of the Erythrophleum guineensis; demonstrated
antimicrobial activity towards seven strains of microorganisms
including three yeasts and four bacteria which known to be involved
in food poisoning and acute infectious diarrhea. Among these, highest
activity has been found against S.
aureus, C. krusei and C.
parapsilosis [12].
In
another study, corilagin has shown a significant synergism with the
antibiotics like cefmetazole, oxacillin, imipenem and
benzylpenicillin against MRSA and also found to be specific with β
lactams by 100- to 2000-fold but little or no synergism with, other
antibiotics like ofloxacin, streptomycin, tetracycline and vancomycin
[13].
Shiota et al. have reported that corilagin and tellimagrandin
I isolated from Arctostaphylos uva-ursi and Rosa canina remarkably
reduced the MIC of β-lactams in MRSA. The mechanistic studies
indicated that inactivation of penicillin-binding proteins (PBPs),
especially of PBP2 (PBP2a), by corilagin or tellimagrandin I was the
major reason for the remarkable reduction in the resistance level of
β-lactams
in MRSA. Furthermore, corilagin and tellimagrandin I also suppressed
the β-lactamase
activity to a small extent [14].
Plant Tannins as an Anti-yeast, Anti-fungal Agent
The peel extract of the plant P. granatum has shown higher antifungal activity against Aspergillus niger [15]. The ethanolic extract of peel and pericarp extract also contain tannins punicalagin and galladydilacton and inhibiting effect on the growth of fungi. The change in the morphology of hyphae and cell membrane in C. albicans and C. krusei, have proven potential of the tannins against Candida genus
[16].
Punicalagin is also found in the aqueous extract of the peels
of P. granatum and the extract shows considerable effects against
Stemphylium botryosum,
Alternaria alternata,
and Fusarium
species. Methanolic extract of pomegranate flower has also shown a
good results against C.
albicans.
In one
study, GTs from unripe mango has expressed potent activity against
anthracnose-resistant strains of Colletotrichum
gloesoporioides and
Alternaria alternate
than the susceptible or non-resistant strains and the activity
diminished on ripening of the fruit [17].
The ETs obtained from Euphorbia antisyphilitica has
antifungal activity against four fungi viz Fusarium
oxyzporum, A. alternata, Colletotrichum gloeosporoides
and Rhizoctnia
[18].
The phenolic extract of C. ladanifer is rich in punicalagin gallate,
and has shown activity against C.
albicans, C. glabrata
and Candida parapsilosis
[19].
In another study, Morey et al. evaluated the effect of CT-rich fraction of Stryphnodendron adstringens on in vitro and in vivo growth of C. tropicalis, and on yeast adhesion properties. The plant tannin-rich fraction exhibited a fungistatic effect with MIC concentration ranging from 0.5 to 8.0 μg/mL with a significant reduction in biofilm mass and reduction in yeast adherence on HEp-2 cells [20].
Mechanism of Action of Plant Tannins
The mechanisms of plant tannin toxicity are generally related to some of their characteristic physicochemical properties, particularly astringency. The addition of a ligand which can compete with microbial ligands such as enzymes can reduce microbial inhibition by tannins. The astringent character of plant tannins may induce complexation with enzymes or substrates. They can directly affect the metabolism by inhibiting oxidative phosphorylation by mitochondria and inhibition of the electron transport chain. They can affect the integrity of cell membranes [21].
Another toxicity mechanism involves the complexation of metal ions by plant tannins. Biological systems, including microorganisms, are highly dependent on the metal ion status of the environment. Antimicrobial activity through iron depletion is particularly well documented. For example, infection of humans by E. coli is subsided by the iron-chelating lactoferrin present in human milk but restored by an excess of iron. Tannins might exert their antimicrobial action through a similar mechanism. Most of the plant tannins have more than two o-diphenol groups in their molecule, which can form chelates with many metal ions such as ferric or cupric ions also [22].
Bioavailability and Metabolism of Plant Tannins
The oral route is unfavorable for HTs as they are hydrolysed in the intestinal pH and have poor pharmacokinetic parameters. Urolithins-the metabolites of ETs-have to be studied for its pharmacological and toxicological effects in detail to ensure the health benefits. Moreover, HTs have higher idiosyncrasy, that is, has variable pharmacokinetic property among the individuals studied. Hence, a suitable oral drug delivery system has to be formulated for HTs to achieve bioavailability. However, the intraperitoneal route, injection, of administration of HTs has proven to be quite efficient because the plasma concentration of ellagic acid (hydrolysed product of ETs) has increased, and the elimination was protracted [2].
Gallotannins (GTs) and Ellagitannins( ETs) are the categories of hydrolysable tannins(HTs). They contain ester bonds and can be hydrolysed, or destroyed, by the acidic pH of the stomach. So they are not effective when taken by mouth.
Condensed plant tannins(CTs) contain flavanoid units joined by carbon-carbon bonds which are much stronger and resistant to hydrolysis and can be effective orally.
Plant Tannins Conclussion
Plant tannins are astringent, bitter-tasting plant polyphenols having sufficient hydroxy and carboxyl groups to form stable complexes with proteins and other macromolecules. Over the last century, studies on plant tannins have moved from organoleptic properties in foods and industrial applications (leather production) to the present-day research into biological properties allied with disease prevention.
These secondary plant metabolites have demonstrated their antimicrobial potential when used alone or in combination with the existing antibiotics. They can interact with bacterial cell walls, metabolic enzymes and essential elements required for survival of microorganisms. Hence, more emphasis should be given to this field since these phytochemicals can act through different mechanisms in comparison to the conventional antibiotics and thus can have the potential in reversing microbial resistance or acting through a pathway different from those of conventional antibiotics.
About the Author

Dr. Harmeet Kaur received her Bachelors in Pharmacy from Guru Nanak Dev University in Amritsar, India in 2000. Guru Nanak Dev University is a state owned university with an "A" grade nationally.
Dr. Kaur received her Masters in Medicinal Chemistry from the National Institute of Pharmaceutical Education and Research in 2002.
In 2015 Dr. Kaur was awarded her Ph.D in Pharmaceutical Sciences from Maharshi Dayanad University in Rohtak, India.
Dr. Kaur is presently a Senior Research Scientist at Maharshi Dayanand University in India.
Dr. Kaur has over 35 published Research papers concerning infectious diseases caused by yeasts, fungi, and bacteria using both prescription drugs and natural plant compounds. She has also performed many studies on cancer cells.
Of particular importance to us, is her multiple experiments performed on Candida albicans and pathogenic bacteria using natural compounds. Because of this experience, we are thrilled to have her on the YeastInfectionAdvisor team.
Back to Herbal Yeast Infection Remedies
Any questions about plant tannins please feel free to contact us from the contact page of this website or talk to your doctor.
Dr. Kaur's References
1. Bele AA, Jadhav VM, Kadam VJ. Potential of tannnins: A review. Asian Journal of Plant Sciences. 2010;9(4):209.
2.
Ekambaram SP, Perumal SS, Balakrishnan A. Scope of hydrolysable tannins
as possible antimicrobial agent. Phytotherapy Research. 2016;30(7):1035-45.
3. Khanbabaee K, van Ree T. Tannins: classification and definition. Natural Product Reports. 2001;18(6):641-9.
4. Laks PE. An overview of condensed tannin structure. InChemistry and significance of condensed tannins 1989 (pp. 131-136). Springer, Boston, MA.
5.
Miyasaki Y, Rabenstein JD, Rhea J, Crouch ML, Mocek UM, Kittell PE,
Morgan MA, Nichols WS, Van Benschoten MM, Hardy WD, Liu GY. Isolation
and characterization of antimicrobial compounds in plant extracts
against multidrug-resistant Acinetobacter baumannii. PloS one. 2013;8(4).
6.
Adesina SK, Idowu O, Ogundaini AO, Oladimeji H, Olugbade TA, Onawunmi
GO, Pais M. Antimicrobial constituents of the leaves of Acalypha
wilkesiana and Acalypha hispida. Phytotherapy Research. 2000;14(5):371-4.
7.
Li N, Luo M, Fu YJ, Zu YG, Wang W, Zhang L, Yao LP, Zhao CJ, Sun Y.
Effect of corilagin on membrane permeability of Escherichia coli,
Staphylococcus aureus and Candida albicans. Phytotherapy Research. 2013;27(10):1517-23.
8.
Taguri T, Tanaka T, Kouno I. Antimicrobial activity of 10 different
plant polyphenols against bacteria causing food-borne disease.
Biological and Pharmaceutical Bulletin. 2004;27(12):1965-9.
9.
Chung KT, Lu Z, Chou MW. Mechanism of inhibition of tannic acid and
related compounds on the growth of intestinal bacteria. Food and
Chemical Toxicology. 1998 ;36(12):1053-60.
10.
Funatogawa K, Hayashi S, Shimomura H, Yoshida T, Hatano T, Ito H, Hirai
Y. Antibacterial activity of hydrolyzable tannins derived from
medicinal plants against Helicobacter pylori. Microbiology and
Immunology. 2004;48(4):251-61.
11.
Maisetta G, Batoni G, Caboni P, Esin S, Rinaldi AC, Zucca P. Tannin
profile, antioxidant properties, and antimicrobial activity of extracts
from two Mediterranean species of parasitic plant Cytinus. BMC
Complementary And Alternative Medicine. 2019;19(1):82.
12.
Joseph N, Mirelle AF, Matchawe C, Patrice DN, Josaphat N. Evaluation of
the antimicrobial activity of tannin extracted from the barks of
Erythrophleum guineensis (Caesalpiniaceae). Journal of Pharmacognosy and
Phytochemistry. 2016;5(4):287.
13.
Shimizu M, Shiota S, Mizushima T. Marked potentiation of activity of
β-lactams against methicillin-resistant Staphylococcus aureus by
corilagin. Antimicrobial Agents and Chemotherapy. 2001; 45:3198–3201.
14.
Shiota S, Shimizu M, Sugiyama JI. Mechanisms of action of corilagin and
tellimagrandin I that remarkably potentiate the activity of β-lactams
against methicillin-resistant Staphylococcus aureus. Microbiology and
Immunology. 2004;48: 67-73.
15.
Dahham SS, Ali MN, Tabassum H, Khan M. Studies on antibacterial and
antifungal activity of pomegranate (Punica granatum L.).
American-Eurasian Journal of Agricultural & Environmental Sciences.2010;9(3):273-81.
16.
Anibal PC, Peixoto IT, Foglio MA, Höfling JF. Antifungal activity of
the ethanolic extracts of Punica granatum L. and evaluation of the
morphological and structural modifications of its compounds upon the
cells of Candida spp. Brazilian Journal of Microbiology. 2013;44(3):839-48.
17.
Karunanayake LC, Adikaram N, Kumarihamy BM, Bandara BR, Abayasekara C.
Role of antifungal gallotannins, resorcinols and chitinases in the
constitutive defence of immature mango (Mangifera indica L.) against
Colletotrichum gloeosporioides. Journal of Phytopathology. 2011;159(10):657-64.
18.
Ascacio-Valdés J, Burboa E, Aguilera-Carbo AF, Aparicio M,
Pérez-Schmidt R, Rodríguez R, Aguilar CN. Antifungal ellagitannin
isolated from Euphorbia antisyphilitica Zucc. Asian Pacific Journal of
Tropical Biomedicine. 2013;3(1):41-6.
19.
Barros L, Dueñas M, Alves CT, Silva S, Henriques M, Santos-Buelga C,
Ferreira IC. Antifungal activity and detailed chemical characterization
of Cistus ladanifer phenolic extracts. Industrial Crops and Products. 2013;41:41-5.
20.
T Morey A, C de Souza F, P Santos J, A Pereira C, D Cardoso J, SC de
Almeida R, A Costa M, CP de Mello J, V Nakamura C, Pinge-Filho P, M
Yamauchi L. Antifungal activity of condensed tannins from
Stryphnodendron adstringens: effect on Candida tropicalis growth and
adhesion properties. Current Pharmaceutical Biotechnology. 2016;17(4):365-75.
21.
Khameneh B, Iranshahy M, Soheili V, Bazzaz BS. Review on plant
antimicrobials: a mechanistic viewpoint. Antimicrobial Resistance &
Infection Control. 2019;8(1):118.
22. Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30(12):3875-83.
Home Privacy Policy Copyright Policy Disclosure Policy Doctors Store
Copyright © 2003 - 2024. All Rights Reserved under USC Title 17. Do not copy
content from the pages of this website without our expressed written consent.
To do so is Plagiarism, Not Fair Use, is Illegal, and a violation of the
The Digital Millennium Copyright Act of 1998.