- Home
- Enzyme Therapy
Do Enzymes For Yeast Infection and Candida Really Work?
Table of Contents
Updated 8/5/2024
Medically Reviewed by Dr. Atmika Paudel, PhD - Written by Dan Jackowiak Nc, HHP and Dr. Vibhuti Rana, PhD
Dr. Atmika Paudel, PhD says... The information in the article written above explaining the
biofilm structures and the importance of enzymes for biofilm and yeast cell wall degradation
is correct.
Enzymes for yeast eat the cell wall of Candida and other yeasts because the cell wall is composed of fiber and glycoproteins. Many times the patient will not experience any die-off reaction at all, especially when using an enzyme that contains protease, which is a huge bonus.
Lets review the basic cell and biofilm structure of yeasts.
Yeast Cell Structure Review
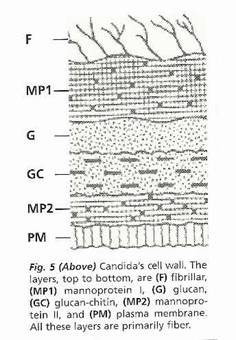
Candida yeast has a cell wall composed of mannoprotein-1, glucan, chitin-glucan, mannoprotein-2 and plasma membrane.
A mannoprotein is a complex carbohydrate-modified protein used by yeasts to anchor the proteins to the cell wall glucans.
Glucans
are a polysaccharide fiber, that are found in the cell walls of
bacteria, fungi, algae, lichens and plants such as oats and barley.
Chitins are long-chain polymer of N-acetylglucosamine, which is a derivative of glucose. It is a primary component of fungal cell walls and the exoskeletons of arthropods. It is very similar to the polysaccharide cellulose - the basic fiber component of plant cell walls.
The plasma membrane is a fatty lipid and protein layer and the nucleus of the yeast cell is also made of protein.
The fatty acids oleic, linolic, palitic, and palmitolic are found throughout its structure.
Knowing that the cell wall of Candida yeast is made up of proteins with carbohydrate rich fibers including a protein and lipid nucleus, a properly designed enzyme formula should contain the necessary enzymes to digest all of these parts.
Dr. Vibhuti Rana, PhD says…
The Candida cell wall is a strong structural feature of the yeast, having high proportions of fibrillar polysaccharides and proteins. These can be adherent proteins (having glyco-phosphatidylinositol linkage), secretory proteins (having N-terminal signal peptide), and another set of proteins for which surface association is unknown. (1)
Moreover, Mishra
and Prasad, in a book published in 1991, discussed that the first step or initiation of fungal infection is by lipid mediated adherence to host cells. Among these, neutral lipids and phospholipids are major constituents. Some of them are triacylglycerol, diacylglycerol, fatty acids, sterols, phosphatidylcholine, phosphatidylethanolamine , phosphatidylserine, and phosphatidylinositol (PI). In addition, some fatty acids that are unique to Candida species are linoleic and linolenic acids, which have varied levels during the different morphological transitions of yeast. (2)
1. W. LaJean Chaffin, Microbiology and Molecular Biology Reviews Sep 2008,72 (3) 495-544; DOI: 10.1128/MMBR.00032-07.
2. Mishra P., Prasad R. (1991) Lipids of Candida albicans. In: Prasad R. (eds) Candida Albicans. Springer, Berlin, Heidelberg.
Yeast Biofilm Structure Review
Biofilms are used by pathogenic yeasts, bacteria and viruses as a way to hide from the hosts immune system. At the same time the pathogen will release spores or cells in an attempt to spread throughout the body. Upon release of the spores the immune system mounts a defense, which causes inflammation and can make you feel sick.
Yeast biofilms generally contain parts of the cell wall structure itself.
It will be composed of cellulose, which is basically a chain of polysaccharide fibers.
The biofilm will also contain polynucleotides that are made from bonded sugars and are primarily its DNA and RNA material.
Also present are polypeptide
proteins and carbohydrate glucan polysaccharides.
The biofilm also contains fibrinogen and fibronectin or scar tissue at the attachment sites, which are the same materials that the body uses to clot the blood and repair wound sites.
All of these materials are bound together by ligands with stickiness properties much like lectins in wheat.
The biofilm will form in the section of the picture above marked F. The biofilm is the first thing you need to get through in order to have any effect on the yeast cell itself.
So, the biofilm is made from proteins, with carbohydrate rich fibers much like the cell wall. In addition, microorganisms such as bacteria and yeast use fibrinogen and fibronectin to adhere and make biofilms with the help of fibronectin binding proteins present in these microorganisms. More on the cell structure and biofilms here.
Dr. Vibhuti Rana, PhD says…
Biofilm is an extracellular matrix, made up of exopolymeric materials, and is a characteristic feature of Candida pathogenesis, the third/fourth most common infection in US hospitals. Biofilm formation is affected by alterations in temperature, pH, growth medium, etc. Proteins constitute more than 55% of the dry mass of biofilm, followed by carbohydrates (25%), lipids (15%), and nucleic acids (5%). (1)
Biofilms enable the pathogens to escape from the host immune response and the drug effects. Biofilms pose a serious problem as they can also grow on medical devices, such as prostheses, cardioverter defibrillators, urinary and vascular catheters, and cardiac devices, further obstructing the elimination of Candida infections. (2)
1. Pierce CG, Vila T, Romo JA, et al. The Candida albicans Biofilm Matrix: Composition, Structure and Function. J Fungi (Basel). 2017;3(1):14. doi:10.3390/jof3010014.
2. Cavalheiro M, Teixeira MC. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front Med (Lausanne). 2018;5:28. Published 2018 Feb 13. doi:10.3389/fmed.2018.00028.
Enzymes Needed for The Best Results
For the mannoproteins in the cell, polypeptide proteins in the biofilm, and the nucleus of the yeast cell you need a protease enzyme.
The
glucans and cellulose-chitin found in both the cell wall and biofilm
you need cellulase and hemicellulase. Beta-glucanase is also very helpful but
a quality cellulase will have beta-glucanase side activity so you're covered in
that respect.
The polysaccharides found in both the cell and biofilm can be removed with amylase, invertase and glucoamylase.
The fibrinogen, fibronection and the ligands in the biofilm can be removed with serrapeptase(serratiopeptidase) and nattokinase.
For the fatty lipids a lipase enzyme works well.
In our opinion, getting through the biofilm is the most important aspect when treating yourself for candida yeast infections. If you don't get through the biofilm, you are not going to have any long term success getting the yeast under control.
What enzyme for yeast brands are the best to use? See our review here
Do Enzymes for Yeast Really Work on the Cell Wall?
Way back in 1968 Roberte Domanski and Ruth Miller from the Dept. of Microbiology at the Women's Medical College of Pennsylvania, used a chitanase complex and beta-glucanase on the cell walls of Candida albicans. Within 5 minutes of exposure to the enzymes in the presence of water, the cell walls burst. Note the intact cells in picture 1 and the disrupted cell in picture 2.
In 1971 Canadian scientist Joseph Brown analyzed the
helicase enzyme from the snail Helix pomatia, effects on the cells of
Torulopsis glabrata, Candida albicans, and Saccharomyces cerevisiae. He
noted that the effects of the enzyme on Candida glabrata wore off in a
couple of hours as the fungi adapted to the effects of the enzyme. It took much longer for the other two yeast cell walls
to adapt to the enzyme.(1)
In 1976 the effects of enzymes
on the cell surface of Candida Albicans was examined at the Department of
Biochemistry, The University, in Leeds. Beta-glucanase worked well but
major changes took place with the addition of chitanase and protease,
which resulted in the greatest disruption of the cell wall.(2)
In
1980, E. F. Gale and associates ran enzyme studies on the cell
structure of Candida albicans to see if they would help reduce the
organisms ability to resist Amphotericin B. They found that
beta-glucanase had the greatest effects on the cell wall. They also
found that the effects were further enhanced when using chitinase,
trypsin - which is a protease, or lipase.(3)
A 2002 medical study evaluated the effects of the enzymes
protease and amyloglucosidase on the yeast Saccharomyces
cerevisiae. It was found
that the protease caused a progressive increase of surface roughness.
Large depressions surrounded by protruding edges were formed and
attributed to the erosion of the mannoprotein outerlayer. The
amyloglucosidase had no effect, which is what they expected based on the
cell structure of Saccharomyces cerevisiae. The study also noted that Saccharomyces cerevisiae is susceptible to protease.(4)
In Sept of
2017, a study was performed in South Korea at the College of
Agricultural and Life Science on the fungi Fusarium oxysporum and
Rhizoctonia solani. Chitanase and Beta-glucanase enzymes were used to
see if they had any effect on the cell wall structure. The enzymes were
found to inhibit
hyphal growth of these two species.(5)
Because Candida yeast is also a fungi with similar cell structure, there is no reason to not believe that these enzymes would not work on hyphael producing strains of Candida.
1. Journal of General Microbiology (1980), 119, 341-349. Printed in Great Britain 341 Protoplasts from Yeast and Mycelial Forms of Candida albicans
2. Journal of General Microbiology (1976),
95,335-347 Printed in Great Britain 335 Changes in the Cell Surface of
the Dimorphic Forms of Candida albicans by Treatment with Hydrolytic
Enzymes.
3. Journal of General Microbiology (1980), 117,
383-391. Printed in Great Britain Reduction of Amphotericin Resistance
in Stationary Phase Cultures of Candida albicans by Treatment with
Enzymes
4. Yeast. 2003 Jan 15;20(1):25-30. Real-time imaging of the surface topography of living yeast cells by atomic force microscopy.
5. Microb Pathog. 2017 Sep;110:159-164.
doi: 10.1016/j.micpath.2017.06.038. Epub 2017 Jun 28. Antifungal
activity and expression patterns of extracellular chitinase and
β-1,3-glucanase in Wickerhamomyces anomalus EG2 treated with chitin and
glucan.
What have we learned from these studies?
- In every study the enzymes did have an effect on the cell wall.
- Using multiple enzymes had the greatest effects.
- Beta-glucanase seems to be the best enzyme but alone is not the answer either. It should be noted, according to our lab testing, a quality cellulase will have beta-glucanase side activity.
- A quality cellulase will also have Chitinase side activitiy.
- Protease does indeed work on the mannoproteins found in the cell walls of yeasts. Its effects can be further enhanced by the addition of cellulases and lipase.
The obvious conclusion, based on the uses of these enzymes singularly and when combined, is to use an enzyme formula that has all the necessary enzymes required to digest every component of the cell wall. The studies suggest that complete formulas are going to have the greatest effects.
Dr. Vibhuti Rana, PhD says...
Biofilms are a hard to remove source of infection caused by yeast pathogens. Anti‐biofilm enzymes can target the cells embedded in the matrix and promote cell lysis. Nonetheless, these enzymes mainly focus on the extracellular polymeric substances (EPS) and break down the cell‐to‐cell connectively, along with degrading the matrix macromolecules. (1)
In the past, disruption of biofilms has been tried by use of acids/bases, or disinfectants, to a negligible benefit. Therefore, enzymatic removal of biofilms remains the main method of these contaminating extracellular polymeric substances (EPS) containing biofilms. Research shows that a selected cocktail of enzymes, isolated from different strains, work best at targeting biofilm matrix. (2) The terpenic derivatives have also shown some promising results in biofilm tackling.
1. Nahar, S., Mizan, M.F.R., Ha, A.J.‐w. and Ha, S.‐D. (2018), Advances and Future Prospects of Enzyme‐Based Biofilm Prevention Approaches in the Food Industry. Comprehensive Reviews in Food Science and Food Safety, 17: 1484-1502. doi:10.1111/1541-4337.12382
2. Gauthier Boels (2011) Enzymatic removal of biofilms: A report, Virulence, 2:5, 490-489, DOI: 10.4161/viru.2.5.17317
Do Enzymes for Yeast Really Work on the Biofilm?
Yes they do and they work extremely well. They also work for bacterial biofilms as we're going to prove to you. This is very important because half of the stool tests we see show no yeast but over growths of pathogenic bacteria.
A published study in 2006 that was done at the Institute of Biomedical and Life Sciences in Glasgow, Uk; used multiple
enzymes to see what effects they had on the biofilms of both Candida
albicans and Candida
tropicalis.
Beta-glucanase more easily detached Candida albicans biofilms from plastic surfaces. Protease, the cellulases, and glucoamylase partially detached the biofilm. Candida tropicalis biofilms were only affected by lipase and the chitinases. They also noted that the biofilms definitely contributed to increased drug resistance.(6)
A 2017 study done at the Medical University in Vienna, Austria on the biofilms of Candida albicans using beta-glucanase only; had no effect on its growth but reduced
the biofilm by 56%. This greatly enhanced the ability of Fluconazole and Amphotericin B to clear the infection.(7)
The same doctors in Vienna ran another study to monitor the effects of beta-glucanase on the biofilms of Candida tropicalis, Candida parapsilosis and Candida krusei.They observed
a reduction in the biofilm by 60%, which greatly enhanced the effects of Amphotericin B. The enzyme had no effect on these species of yeast growth characteristics.(8)
6. J Med Microbiol. 2006 Aug;55(Pt
8):999-1008. Biofilm matrix of Candida albicans and Candida tropicalis:
chemical composition and role in drug resistance.
7. Int J Biol Macromol. 2018 Mar;108:942-946.
doi: 10.1016/j.ijbiomac.2017.11.003. Epub 2017 Nov 4. β-1,3-glucanase
disrupts biofilm formation and increases antifungal susceptibility of
Candida albicans DAY185.
8. Microb Pathog. 2017 Dec;113:342-347.
doi: 10.1016/j.micpath.2017.10.057. Epub 2017 Nov 1. Dispersal of
single and mixed non-albicans Candida species biofilms by
β-1,3-glucanase in vitro. https://www.ncbi.nlm.nih.gov/pubmed/29101060
Enzyme Effects on Bacterial Biofilms?
Bacterial biofilms are composed of proteins, lipids, polysaccharides, and extracellular DNA. The exact amounts will vary slightly depending upon the bacteria that created them.
This scientific study
done by Loiselle M, Anderson KW at the Department of Chemical and
Materials Engineering, University of Kentucky, on the biofilm of the
pathogenic bacteria Pseudomonas aeruginosa using the cellulase enzyme,
showed a marked reduction in the film. The authors noted that using
additional enzymes with the cellulase would probably increase the
effectiveness, which is what we have suggested all along.
In
2011, the Max Bergmann Center of Biomaterials in Dresden Germany did
a medical study on the biofilms of Pseudomonas aeruginosa and Staphylococcus
epidermidis. They used glycoside hydrolase's, which include the enzymes
cellulase, hemicellulase, and amylase. They also used subtilisin A, which
is a protease enzyme derived from the bacteria Bacillus subtilis.
They
observed that cellulase reduced the biofilm of Staphylococcus
epidermidis by 67% while having no effect on the biofilm of Pseudomonas
aeruginosa. The protease enzyme had the opposite effect, it had no
effect on the biofilm of Staphylococcus epidermidis but reduced the
biofilm of Pseudomonas aeruginosa by 44%.(9)
A 2013 study on
the bacteria Burkholderia cepacia that typically infects people with
medical implants, was done at the Department of Microbiology, PRIST
University in India. They used the cellulase enzyme and noted "significant
anti-biofilm activity".(10)
In 2017, a study was
performed at the University of Porto in Portugal on the biofilms of the
pathogenic bacteria Pseudomonas fluorescens. The enzyme beta-glucanase,
amylase, lipase and protease were used both by themselves and in
combination. A
modest removal of the biofilm was observed with protease
having the greatest long term effects.(11)
Another 2017
study done at Institute of Fundamental Medicine and Biology in Russia,
tested protease on the biofilms of the Staphylococcus aureus and
Staphylococcus epidermidis bacteria. Biofilm
thickness decreased by 200% within 24 hours of exposure to the protease. The amount of antibiotics
then needed to clear the bacteria decreased three fold.(12)
9. Biotechnol Lett. 2011 Sep;33(9):1897-904.
doi: 10.1007/s10529-011-0643-3. Epub 2011 May 27. Immobilized enzymes
affect biofilm formation. https://www.ncbi.nlm.nih.gov/pubmed/21618024
10. Pol J Microbiol. 2013;62(3):327-30.
Cellulase inhibits Burkholderia cepacia biofilms on diverse prosthetic
materials. https://www.ncbi.nlm.nih.gov/pubmed/24459841
11. Food Res Int. 2017 May;95:101-107.
doi: 10.1016/j.foodres.2017.02.016. Epub 2017 Feb 28. Combination of
selected enzymes with cetyltrimethylammonium bromide in biofilm
inactivation, removal and regrowth.
https://www.ncbi.nlm.nih.gov/pubmed/28395817
12. Sci Rep. 2017 Apr 7;7:46068. doi: 10.1038/srep46068. Targeting microbial biofilms using Ficin, a nonspecific plant protease.
Dr. Vibhuti Rana, PhD says...
By now, it is quite clear that a biofilm provides a three-dimensional shield to the cells inside it, be it bacterial, fungal or viral. The chief requirement is to remove or disperse these biofilms using enzymes that are best suited for this job.
Reports have revealed that bacterial polysaccharides are alginate (by Pseudomonas aeruginosa) and poly β 1, 6-linked N-acetylglucosamine (produced by S. epidermidis and S. aureus). This report conducted enzymatic degradation assay on C. albicans and C. tropicalis biofilm matrix using eight different enzymes. Resultantly, it was seen that proteinase K, chitinase, DNase I or β-N-acetylglucosaminidase enzymes were able to partially degrade matrix material, leading to biofilm detachment from the well surfaces. The enzyme lyticase exhibited the greatest effect, causing an 85 % reduction in optical density of the biofilm (1)
In another study, the enzymes lyticase and beta-glucosidase have been shown to significantly remove Pseudomonas aeruginosa bacterial biofilms, without causing any toxic side-effects in host. (2) In Staphylococcus aureus forming biofilms, enzyme debridement has been reported using enzymatic agents such as papain/urea, bromelain, DNase I/fibrolysin, krillase, dispersin, Proteinase-K, sutilains, and collagenase. (3)
1. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance Journal of Medical Microbiology (2006), 55, 999–1008
2. Banar, M., Emaneini, M., Beigverdi, R. et al. The efficacy of lyticase and β-glucosidase enzymes on biofilm degradation of Pseudomonas aeruginosa strains with different gene profiles. BMC Microbiol 19, 291 (2019).
3. Watters CM, Burton T, Kirui DK, Millenbaugh NJ. Enzymatic degradation of in vitro Staphylococcus aureus biofilms supplemented with human plasma. Infect Drug Resist. 2016;9:71–78. Published 2016 Apr 27. doi:10.2147/IDR.S103101.
Best Enzyme for the Fibrinogen and Fibronectin in the Yeast Biofilm
Dr. Atmika Paudel, PhD says... "Microorganisms such as bacteria and yeast use fibrinogen and fibronectin to adhere and make biofilms with the help of fibronectin binding proteins present in these microorganisms."
Besides being a very good anti-inflammatory agent, serratiopeptidase literally eats these substances which are basically scar tissue.
Because this enzyme digests non-living tissue and leaves live tissue alone; it has been found to be effective in removing the deposits of fatty substances, cholesterol, cellular waste products, calcium and fibrin on the inside of the arteries. The fibrinolytic clot removing activity of serratiopeptidase may also be able to help with thickened blood, increased risk of stroke, and phlebitis/thrombophlebitis.
It's been reported to have an effect on sinusitis and bronchitis, atherosclerosis, carpal tunnel syndrome, rheumatoid arthritis and other autoimmune diseases.
Serrapeptase effects on biofilms
A 1993 study on prosthetic joints infected by Pseudomonas aeruginosa and Staphylococcus epidermidis, found that serrapeptase greatly enhanced the activity of the antibiotic ofloxacin against these pathogens by inhibiting
and removing biofilm formation.(13)
A 2008 study that was published in Microbial Pathogenisis was done on the pathogenic bacteria Listeria monocytogenes, which is a bacteria that causes food poisoning, found that serratiopeptidase
sharply reduced the bacteria's ability to form biofilms and attach itself to the human gut.(14)
Serrapeptase
has been shown to inhibit fibrin from growing and becoming problematic in an induced animal model, which enhanced the penetration of antibiotics to sites of infection.(15)
In another study, five different protease enzymes were tested on Staphylococcus aureus and Staphylococcus epidermidis bacteria strains for their antimicrobial efficiency. Some proteases showed a nonspecific and indiscriminate effect on the proteins in the biofilm, while others induced a discrete and reproducible action on the proteins. The study concluded that serratiopeptidase,
can interfere with adhesion and invasion of eukaryotic cells and
biofilm formation.(16)
The ability of serrapeptase to inhibit Staphylococcus aureus's ability to invade human tissues and to adhere to prostheses, catheters and medical devices was proven
again in a separate 2013 study.(17)
In 2006, Serratiopeptidase was effective for eradicating infection caused by biofilm-forming bacteria in an experimental animal model.(18)
In spite of being in use for decades, serrapeptase has not been given GRAS status by the FDA but is licensed for use as a natural health product by Health Canada.
Doing a quick search on the National Library of Medicine for nattokinase and biofilms we find six studies proving its effectiveness against biofilms produced by various species of bacteria.
WebMD suggests nattokinase helps dissolve blood clots, can lower blood pressure, helps clear nasal passages, improves gut health, and might also help boost your metabolism helping to prevent obesity and diabetes.
I could go on and on here folks but I think at this point, you get it. Especially when you consider these enzymes have been studied repeatedly, and have been found to be effective, since 1968.
13. Selan L, Berlutti F, Passariello C,
Comodi-Ballanti MR, Thaller MC. Proteolytic enzymes: a new treatment
strategy for prosthetic infections? Antimicrob Agents Chemother. 1993;37(12):2618-2621.8109925
14.
Longhi C, Scoarughi GL, Poggiali F, et al. Protease treatment affects
both invasion ability and biofilm formation in Listeria monocytogenes.
Microb Pathog. 2008;45(1):45-52.18479885
15. Kakinuma A,
Moriya N, Kawahara K, Sugino H. Repression of fibrinolysis in scalded
rats by administration of Serratia protease. Biochem Pharmacol. 1982;31(18):2861-2866.6753849
16.
Artini M, Papa R, Scoarughi GL, et al. Comparison of the action of
different proteases on virulence properties related to the
staphylococcal surface. J Appl Microbiol. 2013;114(1):266-277.23057709
17.
Papa R, Artini M, Cellini A, et al. A new anti-infective strategy to
reduce the spreading of antibiotic resistance by the action on
adhesion-mediated virulence factors in Staphylococcus aureus. Microb
Pathog. 2013;63:44-53.23811076
18. Mecitoglu M, Saygi B,
Yildirim Y, Karadag-Saygi E, Ramadan SS, Esemenli T. The effect of
proteolytic enzyme serratiopeptidase in the treatment of experimental
implant-related infection. J Bone Joint Surg Am. 2006;88(6):1208-1214.16757752
What have we learned from these biofilm studies?
- No single enzyme works on the biofilms of every species of candida or bacteria.
- Using multiple enzymes at once enhances the biofilm removing effects.
- Once
the biofilm is lessened or removed, medications are much more
effective. If you were taking prescription drugs such as Fluconazole,
these enzymes would enhance its effects.
- If medications are more effective after biofilm removal, the appropriate herbs or probiotics would be as well.
- After the biofilm has been reduced or removed, the enzymes don't always remove the yeast and definitely not bacteria. That means the best thing to do is to take things that will with these enzymes.
What's the Best way to take these enzymes? See our 3-Step Treatment Plan here.
Summary by Dr. Vibhuti Rana, PhD
As we have already come to know, biofilms
provide a 3-dimensional surface covering mesh comprising of extra
cellular matrix and all macromolecules like proteins, carbohydrates,
lipids, and nucleic acids. The Candida yeast biofilm requires both
yeast-form and hyphal form. The cohesion builds up with gradual
accumulation of extracellular matrix, once the adhesion, cell
multiplication, and hyphal induction steps are completed. (1) The fungal
mass caused by Aspergillus
spp is known as Aspergilloma, and reach
mature stage within 24 hours. (2)
Definitely, as Dan writes, a
cocktail or a combination of different biofilm-digesting enzymes work
best to dilute the density of the biofilm; upon which, suitable
anti-fungal treatment is required to accelerate the process of
decontamination.
The serratiopeptidase metalloprotease enzyme
that belongs to trypsin family has been long known for its
anti-infective properties. It is a potent potent
anti-inflammatory agent used
for treatment of various disorders like bronchitis, arthritis,
sinusitis, carpal-tunnel syndrome, etc. (3) However, literature also
reveals that they have been used to block
biofilm formation and the colonization of S. aureus.
(4)
1. Fanning S, Mitchell AP (2012)
Fungal Biofilms. PLoS Pathog 8(4): e1002585. Published: April 5,
2012 https://doi.org/10.1371/journal.ppat.1002585
https://doi.org/10.1371/journal.ppat.1002585.
2. Costa-Orlandi CB, Sardi JCO, Pitangui NS, et al. Fungal Biofilms and Polymicrobial Diseases. J Fungi (Basel). 2017;3(2):22. Published 2017 May 10. doi:10.3390/jof3020022
3.
The role of serratiopeptidase in the resolution of inflammation. Asian
Journal of Pharmaceutical Sciences. Volume 12, Issue 3, May 2017, Pages 209-215
4. Serratiopeptidase: a well-known metalloprotease with a new non-proteolytic activity against S. aureus biofilm. BMC Microbio. 2015.
Medical Review by Dr. Atmika Paudel, PhD
I have already given my commentary regarding yeast enzyme Biofase and others. This is a similar article that talks about the yeast enzyme components and their respective roles.
This is a similar article that talks about the yeast enzyme components and their respective roles. In addition, the above article gives a detail about the yeast cell structure and its components along with the components of plasma membrane.
Basically, cell wall are made up of carbohydrates and proteins, and play a vital role in recognition of immune cells inside the host, and adhesion to the host cells. The information about the components of Candida cell structure are correct.
An article that was published in 2012 in Current Opinion in Microbiology gives a detail information about how commensal Candida can turn into a pathogen and how its cell structure help it in this transition to establish infection and express virulence factors. (1)
There is a paper published in 2018 in Genome Biology and Evolution that details about a hypervirulent strain of Candida and its genomic comparison with other Candida to give an idea about its evolution. It has been shown that the particular strain harbors many genes related to pathogenesis(2)
Yet another paper published in 2017 in Journal of Fungi gives a detail about the composition of the biofilms of Candida. Biofilms are also composed of carbohydrates and proteins in addition to lipids and nucleic acids. By forming biofilms, Candida hides itself into the protection of the biofilm and becomes resistant to many antifungal agents.
1. Current Opinion in Microbiology, Volume 15, Issue 4, August 2012, Pages 406-412. Importance of the Candida albicans cell wall during commensalism and infection.
2.
Suresh Panthee, Hiroshi Hamamoto, Sanae A Ishijima, Atmika Paudel,
Kazuhisa Sekimizu, Utilization of Hybrid Assembly Approach to Determine
the Genome of an Opportunistic Pathogenic Fungus, Candida albicans TIMM
1768, Genome Biology and Evolution, Volume 10, Issue 8, August 2018, Pages 2017–2022
What enzyme for yeast brands are the best to use? See our review here
Have Any Questions About Enzymes for Yeast?
Do you have any questions about enzymes for yeast or yeast infections in general? Ask your question here or contact us using the contact page of this website. It is also always a good idea to talk to your doctor as well.
Questions From Other Visitors
Click below to see questions from other visitors to this page...
Biofase (systemic enzymes) dosage after 8 weeks 




I have a question about the dosage for taking Biofase. I have candidiasis for 4 years, and leaky gut little less long. I could say it is severe, but I …
How many hours do we have to wait to eat food after we’ve taken enzymes? 




Do I need to take glutamine powder, collagen powder, and lemon juice and food at least 90 minutes away from enzymes so they can work on Candida instead …
Do enzymes harm normal biofilms? 




For example biofilms in the stomach protect cells from acid, and biofilms in the intestine help to lubricate the passage of food.
I am confused on what product to take for my yeast infection? 




I am confused on what product to take for my yeast infection? I have yeast and candida all the time in my pap smear. Now I think I have candida on my toe …
If you have any questions about these enzymes for yeast infection please feel free to contact us from the contact page of this website or talk to your doctor.
Home Privacy Policy Copyright Policy Disclosure Policy Doctors Store
Copyright © 2003 - 2024. All Rights Reserved under USC Title 17. Do not copy
content from the pages of this website without our expressed written consent.
To do so is Plagiarism, Not Fair Use, is Illegal, and a violation of the
The Digital Millennium Copyright Act of 1998.