- Home
- Species & Infections
- Candida Parapsilosis
Candida parapsilosis - An Emerging Human Pathogen
Updated 5/12/2024
Written by Molecular Biologist Dr. Vibhuti Rana, PhD
The invasive candidal diseases caused by the emerging pathogens such as Candida parapsilosis have resulted in serious morbidity and mortality all over the world. Adequate information is available in context to the pathogenic Candida albicans and how it affects us. Although C. albicans remains the leading contributor towards fungal invasive Candida infections, non-C. albicans Candida species like Candida parapsilosis also demand attention owing to their rising pathogenicity. Let’s learn more about this species.
What is Candida parapsilosis?
Candida parapsilosis is one of the leading causes of candidiasis and invasive candidemia. It was first isolated in 1928 from patient stool and termed as Monilia parapsilosis in Puerto Rico. It was also discovered to be the cause of life threatening endocarditis in a drug user (1). Before this, it was regarded as a harmless microbe with no serious health implications.
As of now, Candida parapsilosis has even surpassed the pathogenicity of C. albicans in some European, Brazilian, and Japanese hospitals, where it has been found to exist in very high proportions in the infected blood cultures (2, 3, 4). In a report by Nakamura et al., C. parapsilosis infections were found in appx. 40% of patients while C. albicans accounted for only 30%. Moreover, the former fungi were also resistant to the six major anti-fungal agents tested in the study (4).
Candida parapsilosis has a diploid genome and is not capable of producing true hyphae. Instead, it is characterized by ‘giant cells’, which are actually large, curved pseudohyphae. It forms white, shiny colonies on the Sabouraud dextrose agar (SDA) agar, unlike the bluish green ones produced by C. albicans (5). The appearance of these colonies differs between the pseudohyphae and yeast forms. While the yeast colonies are smooth or crater-like in morphology, the pseudohyphae colonies are rough, crepe-like, or concentric (6). If you are wondering why C. parapsilosis has different colony morphologies, it is due to its different phenotypic forms (a phenomenon known as phenotypic switching) in different isolates. The smooth phenotype contributes the least to biofilm formation and is the least invasive when compared to the others. Among the rest of the phenotypes, the concentric colonies form the highest levels of biofilms on biotic as well as abiotic surfaces (6). It was also been found that C. parapsilosis has a differential capacity to assimilate and ferment different carbon sources: it ferments glucose strongly, galactose moderately, sucrose/maltose weakly, and does not ferment lactose at all (7).
Candida orthopsilosis and Candida metapsilosis, together with Candida parapsilosis sensu stricto compose the C. parapsilosis sensu lato species complex. The former two species are relatively less frequently associated with serious or fatal fungal infections. Nonetheless, they may cause infections of varying degrees in patients with a challenged immune system, or having co-existing immune dysfunction (8).
Virulence of C. parapsilosis
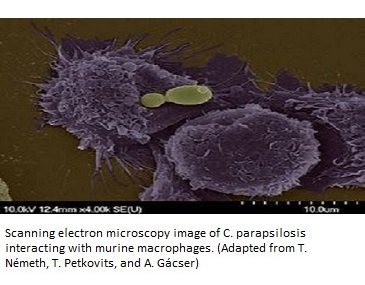
Candida parapsilosis is second only to C. albicans in terms of its infectious capacity. Invasive candidiasis is a key feature of infection by C. parapsilosis. In this condition, different organs of your body including brain, lungs, kidneys, etc. are affected by yeast infection mediated by the blood (Candidemia). This pathogen attacks the human body by disrupting its innate immunity and exploiting the host nutrients and resources for its own growth. The main mode of attack which this yeast species has is the “Candidaparapsins.” These are a group of three aspartic protease enzymes SAPP1p, SAPP2p, and SAPP3p, which are coded by SAPP1, SAPP2, and SAPP3, respectively (9). These enzymes have differential substrate specificity. Other invasive enzymes include lipases (CpLIP2 and CpLIP2 encoded) and phospholipases. All these enzymes basically degrade and denature keratin, collagen, hemoglobin, and serum albumin (9). Role of lipases became clear when a decreased virulence and biofilm formation was observed in the C. parapsilosis strains upon deletion of CpLIP2 and CpLIP2 genes (10).
Apart from these degradation enzymes secreted by C. parapsilosis, factors such as adhesion to host cells, biofilm formation, and modulation of cytokine response are responsible for the invasion of host immunity. A recent study published in Nature, Scientific Reports noted that pathogenic fungi like C. parapsilosis have been seen to utilize and compete with the host iron resources for their own viability. This study also pointed that factors like oxidative stress and temperature also regulate fungal viability and virulence (11).
It is well known that Candida species form biofilms for attachment to medical devices, catheters, etc. where clusters of yeast cells adhere to these surfaces. Biofilms are the primary cause by which C. parapsilosis evades anti-fungal treatment and makes the host cells inaccessible to drug action. Oliveira et al published an interesting research in 2010 in the journal Micron. This study used electron microscopy to show that biofilms by C. parapsilosis have been found on the natural human nail and hair surfaces (12).
Biofilms were seen to be formed equally for all the three species of the C. parapsilosis complex i.e., Candida orthopsilosis, Candida metapsilosis, and Candida parapsilosis (13). It was discovered that C. parapsilosis biofilm layer comprised of irregularly clustered blastospore aggregates. Studies have also reported that in comparison to C. albicans biofilms, C. parapsilosis shows roughly 21% greater avidity to buccal epithelial cells and 143.7% increased adhesiveness to acrylic surfaces used in medical settings (14). The mechanisms of biofilm formation are different between the two species. While C. albicans forms hyphae and penetrates host, subsequently producing biofilm, C. parapsilosis does not form true hyphae. Its pseudohyphae and yeast cells contribute to larger biofilm amounts. As far as similarities are concerned, they are both inhibited by farnesol treatment.
Unlike C. albicans, pathogenic C. parapsilosis has been isolated from non-human hosts such as domesticated animals, soil samples, and aquatic/marine environments of Florida, Bahamas, and even the Indian Ocean (7). C. parapsilosis was found to be one of the non-pigmented yeast species isolated from the deep sea hydrothermal systems of the Portuguese Mid-Atlantic Ridge using the molecular techniques of mini-satellite-primed Polymerase Chain Reaction (MSP-PCR) (15). In 2014, a joint collaborative novel study by the Royal (Dick) School of Veterinary Studies, Scotland, and International Zoo Veterinary Group Pathology, UK, discovered C. parapsilosis to be the cause of death in a 18-month old ferret with accompanying complications like encephalitis, lymphadenitis, and ulcerative dermatitis (16).
Who Are Likely to Develop C. parapsilosis Infections?
Candida parapsilosis generally lives as a commensal (without any harm) on human skin cells. But, when out of control, it infects catheters and other medical devices by forming resilient biofilms on them. The same reason allows it to spread easily via nosocomial (hospital-acquired) infections and makes it highly contagious in hospital settings. It is very commonly acquired by new-born babies who are born underweight or admitted in the neonatal intensive care units (NICU) and account for the highest neonatal death rates due to fungal infections (17, 18). This survey was conducted in 130,523 patients admitted to NICU who were born with a birth weight of one kilogram or less by the National Nosocomial Infections Surveillance system of the USA. The predominant fungal species found to be infecting the blood streams these neonates were C. albicans, C. parapsilosis, and C. glabrata, in addition to C. tropicalis and C. krusei.
Besides, Candida parapsilosis is more likely to infect the skin when it is broken. It means if the skin is wounded, and has cuts or bruises, it is more vulnerable to get infected with this yeast. This also means that it can spread easily through hands and touch (1).
The patients with dysfunctional kidneys (renal failure) are at a greater risk of developing fungal infections. This was shown in a study where the members of the C. parapsilosis complex species re-classified and tested against the most commonly used amphotericin B and fluconazole in 100 environmental samples from a hemodialysis unit. It was found that 72% samples formed biofilms that exhibited resistance to these drugs, making it very important to check the purity of the water used for hemodialysis (19).
Other groups of people who have a risk of developing moderate to severe infections through this yeast are those who have undergone transplants, who have catheters or other implanted prosthetic devices (arthritis related joint infections) in their bodies, artificial heart valves (endocarditis), etc. People undergoing treatment for tuberculosis, HIV, cancer, and other autoimmune disorders are also more likely to catch C. parapsilosis from hospital/medical settings as compared to healthy controls. Such people are also given immunosuppressants, making their bodies all the more vulnerable due to a weakened immune system which is unable to fight off even simple infections on its own. It is also found to be a cause of recurrent endophthalmitis, keratopathy (in cornea implants), and keratitis after LASIK surgeries (1).
Treating Candida parapsilosis Infections
No specific treatment regiment exists to treat fungal infections caused by Candida parapsilosis since it causes a broad spectrum of infections. So, the treatment should ideally comprise of a broad range of drugs or anti-fungals. According to the Infectious Diseases Society of America, amphotericin B–based preparations, the azole antifungal agents, and the echinocandin antifungal agents are the most commonly used treatments for candidiasis (20).
In another study in two neonatal patients, amphotericin B and voriconazole were found to successfully treat invasive candidiasis by this pathogenic species (21).
In each case, it is extremely important to make a profile of drug susceptibility to the different classes of drugs.
To learn how to treat Candida parapsilosis naturally click here.
About the Author

Dr. Vibhuti Rana completed her Bachelors's Degree (Bioinformatics
Hons.) from Punjab University and accomplished her Master’s Degree
(2012) in Genomics with a Gold Medal from Madurai Kamaraj University,
India. In 2020, she received her doctorate in Molecular Biology from the
Council of Scientific and Industrial Research-Institute of Microbial
Technology in affiliation with the Jawaharlal Nehru University, New
Delhi, India.
Her focus areas include microbial drug resistance, epidemiology, and protein-protein interactions in infectious diseases. As a Molecular Biologist with extensive experience with infectious diseases, we are happy she is part of the YeastInfectionAdvisor team.
Any questions about Candida parapsilosis or yeast infections in general? Please feel free to contact us from the contact page of this website or talk to your doctor.
Dr. Rana's Medical References
- Trofa D, Gácser A, Nosanchuk JD. Candia parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008; 21(4):606-625. doi:10.1128/CMR.00013-08. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2570155/)
- Pfaller MA, Diekema DJ, Jones RN, et al. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J Clin Microbiol. 2001; 39(9):3254-3259. doi:10.1128/jcm.39.9.3254-3259.2001. https://pubmed.ncbi.nlm.nih.gov/11526159/
- Medrano DJ, Brilhante RS, Cordeiro Rde A, Rocha MF, Rabenhorst SH, Sidrim JJ. Candidemia in a Brazilian hospital: the importance of Candia parapsilosis. Rev Inst Med Trop Sao Paulo. 2006; 48(1):17-20. doi:10.1590/s0036-46652006000100004. https://pubmed.ncbi.nlm.nih.gov/16547574/
- Nakamura T, Takahashi H. Epidemiological study of Candida infections in blood: susceptibilities of Candida spp. to antifungal agents, and clinical features associated with the candidemia. J Infect Chemother. 2006;12(3):132-138. doi:10.1007/s10156-006-0438-y. https://pubmed.ncbi.nlm.nih.gov/16826345/
- Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, Candia parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance, FEMS Microbiology Reviews, Volume 36, Issue 2, March 2012, Pages 288–305, https://doi.org/10.1111/j.1574-6976.2011.00278.x. https://academic.oup.com/femsre/article/36/2/288/563981
- Laffey SF, Butler G. Phenotype switching affects biofilm formation by Candia parapsilosis. Microbiology. 2005; 151(Pt 4):1073-1081. doi:10.1099/mic.0.27739-0. https://pubmed.ncbi.nlm.nih.gov/15817776/
- Fell JW, Meyer SA. Systematics of yeast species in the Candia parapsilosis group. Mycopathol Mycol Appl. 1967; 32(3):177-193. doi:10.1007/BF02049795 https://pubmed.ncbi.nlm.nih.gov/6051845/
- Nemeth TM , Gacser A, Nosanchuk JD, Candida psilosis Complex, Reference Module in Life Sciences, Elsevier, 2018. https://www.sciencedirect.com/science/article/pii/B9780128096338207097.
- Iva Pichová, Chapter 34 - Candidaparapsin, Handbook of Proteolytic Enzymes (Third Edition), Academic Press, 2013, Pages 166-169 https://www.sciencedirect.com/science/article/pii/B978012382219200034X
- Singaravelu K, Gácser A, Nosanchuk JD. Genetic determinants of virulence - Candia parapsilosis. Rev Iberoam Micol. 2014; 31(1):16-21. doi:10.1016/j.riam.2013.09.018. https://www.elsevier.es/es-revista-revista-iberoamericana-micologia-290-pdf-S1130140613001150
- Tóth R, Nose J, Mora-Montes HM, Gabaldon T, Bliss JM, Nosanchuk JD, Turner SA, Butler G, Vágvölgyi C, Gácser A. Candia parapsilosis: from Genes to the Bedside. Clinical Microbiology Reviews Feb 2019, 32 (2) e00111-18; DOI: 10.1128/CMR.00111-18. https://cmr.asm.org/content/32/2/e00111-18.
- Oliveira MT, Specian AF, Andrade CG, França EJ, Furlaneto-Maia L, Furlaneto MC. Interaction of Candia parapsilosis isolates with human hair and nail surfaces revealed by scanning electron microscopy analysis. Micron. 2010; 41(6):604-608. doi:10.1016/j.micron.2010.03.011. https://pubmed.ncbi.nlm.nih.gov/20430635/
- Lattif AA, Mukherjee PK, Chandra J, et al. Characterization of biofilms formed by Candida parapsilosis, C. metapsilosis, and C. orthopsilosis. Int J Med Microbiol. 2010;300(4):265-270. doi:10.1016/j.ijmm.2009.09.001. https://pubmed.ncbi.nlm.nih.gov/19932053/
- Panagoda GJ, Ellepola AN, Samaranayake LP. Adhesion of Candida parapsilosis to epithelial and acrylic surfaces correlates with cell surface hydrophobicity. Mycoses. 2001; 44(1-2):29-35. doi:10.1046/j.1439-0507.2001.00611.x. https://pubmed.ncbi.nlm.nih.gov/11398638/
- Gadanho M, Sampaio JP. Occurrence and diversity of yeasts in the mid-atlantic ridge hydrothermal fields near the Azores Archipelago. Microb Ecol. 2005;50(3):408-417. doi:10.1007/s00248-005-0195-y. https://pubmed.ncbi.nlm.nih.gov/16328655/
- Mancinelli E, Meredith AL, Stidworthy MF. Systemic Infection Due to Candida parapsilosis in a Domestic Ferret (Mustela putorius furo). J Exot Pet Med. 2014;23(1):85-90. doi:10.1053/j.jepm.2013.11.013. https://pubmed.ncbi.nlm.nih.gov/32288679/
- Levy I, Rubin LG, Vasishtha S, Tucci V, Sood SK. Emergence of Candia parapsilosis as the predominant species causing candidemia in children. Clin Infect Dis. 1998; 26(5):1086-1088. doi:10.1086/520277. https://pubmed.ncbi.nlm.nih.gov/9597232/
- Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan T. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995-2004. Pediatrics. 2006; 117(5):1680-1687. doi:10.1542/peds.2005-1996. https://pubmed.ncbi.nlm.nih.gov/16651324/
- Pires RH, Santos, JM dos, Zaia JE, Martins CHG, and Mendes-Giannini MJS (2011). Candia parapsilosis complex water isolates from a haemodialysis unit: biofilm production and in vitro evaluation of the use of clinical antifungals. Memórias do Instituto Oswaldo Cruz, 106(6), 646-654. https://doi.org/10.1590/S0074-02762011000600002.https://www.scielo.br/scielo.php?script=sci_arttext&pid=S0074-02762011000600002
- Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, Walsh TJ, Edwards JE, Guidelines for Treatment of Candidiasis, Clinical Infectious Diseases, Volume 38, Issue 2, 15 January 2004, Pages 161–189, https://doi.org/10.1086/380796. https://academic.oup.com/cid/article/38/2/161/286280
- Altuncu E, Bilgen H, Soysal A, Ozek E. Successful Treatment of Candida parapsilosis Fungemia in Two Preterms with Voriconazole. Case Rep Pediatr. 2015; 2015:402137. doi:10.1155/2015/402137. https://pubmed.ncbi.nlm.nih.gov/26146582/
Home Privacy Policy Copyright Policy Disclosure Policy Doctors Store
Copyright © 2003 - 2024. All Rights Reserved under USC Title 17. Do not copy
content from the pages of this website without our expressed written consent.
To do so is Plagiarism, Not Fair Use, is Illegal, and a violation of the
The Digital Millennium Copyright Act of 1998.